Become an Expert at
Vedak
Expand
Access 625k+ Domain Experts with Vedak
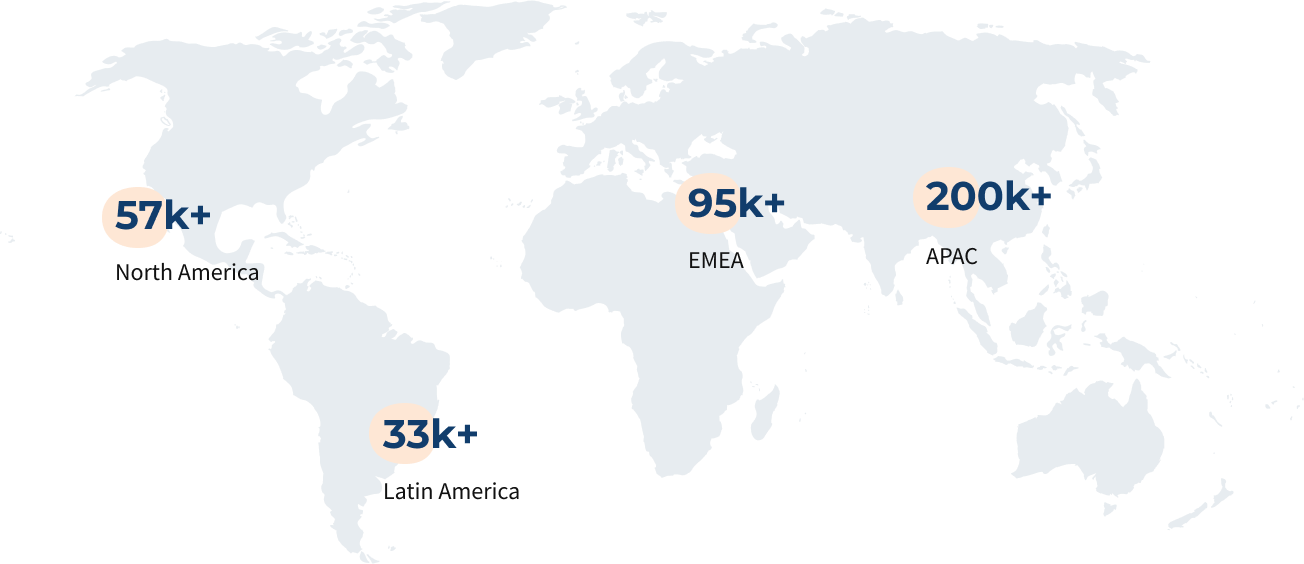
Expertise Across Industries
Banking & Financial Services
Consumer, Retail & E-Commerce
Healthcare & Hospital Management
Pharmaceuticals & Medical Products
Travel, Transport & Logistics
Manufacturing & Automobile
Real Estate And Construction
Education & Training

Grow Your Expertise With Vedak
Become An Expert With Vedak And Be A Part Of Our Exclusive Global Network
Join Vedak and grow your expertise across diverse industries. As a member, you’ll gain access to rewarding projects with leading names in your industry, receive guaranteed payments, and enjoy 24/7 support from us. Connect with a global community of professionals and elevate your career while making meaningful contributions to the growth of our clients.
Meet Our Expert
Our Pool Of Industry Experts
Register Now
How To Become A Vedak Experts
Registration
Get contextualized advice from world’s leading experts in an easy to use per-hour format 12500+ Expert Calls Completed
Profile Creation
Get contextualized advice from world’s leading experts in an easy to use per-hour format 12500+ Expert Calls Completed
Engagement
Get contextualized advice from world’s leading experts in an easy to use per-hour format 12500+ Expert Calls Completed
Let’s connect
